Reading Time – 8 minutes, Difficulty Level 4/5
In this post we are going to examine and try to understand one of the more well established aspects of particle physics, the Heisenberg Uncertainty Principle.
It was first postulated by German physicist Werner Heisenberg in the late 1920’s. It’s name and underlying message were introduced firmly into pop culture more recently in the TV show ‘Breaking Bad’ where lead protagonist Walter White chose the name ‘Heisenberg’ as his pseudonym.
Let’s see if we can understand both the principle itself and why Walter might have cleverly chosen it to represent his character.
Small is beautiful
The uncertainty principle is a scientific fact of the natural world, albeit the natural world of incredibly small things. We call these small things ‘quantum objects’.
A quantum object is any object that cannot be defined by standard physical laws and must be defined by the laws of quantum mechanics. Typically an object that can only be described by quantum mechanics is 1 nano metre in size or smaller, that’s 0.000000001 metres or one billionth of a metre. Pretty small. We would write that size scientifically like this 1×10−9 m.
Things this small behave extremely strangely indeed and we find it incredibly difficult to make accurate predictions about them, most notably their position or ‘place’ and their momentum or ‘speed’.
The equation
Heisenberg’s uncertainty principle is represented by an equation, which I’ve written below. It doesn’t matter too much that we don’t fully understand all the terms precisely. We don’t have to, to be able to gain a high level understanding of it.
Let’s look at it anyway, its does us good to gain more exposure to these equations as we try to build our knowledge and understanding of these subjects.
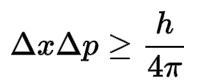
You would read this equation as follows: Delta X multiplied by Delta P is greater than or equal to h over 4 pi.
It looks and sounds complicated but we only need to understand some of it to get the gist.
Delta X is uncertainty in position, Delta P is uncertainty in momentum. The ‘h’ term is something called ‘Planck’s Constant’. This is a very small unit of measurement close to zero. It saves us writing 0.0000000000 and so on over and over again, making the equation even more daunting looking!
The little triangle is the Greek letter ‘Delta’ and in physics we use it to represent a change.
Waves
We’re all pretty familiar with waves, at least the ones we see on water anyway so lets visualise one of those and draw a representation it below.

Duality, two things at the same time
One of the key things to try and understand in this topic is that quantum objects have a property called ‘wave particle duality’. This means they are both a wave and a particle at the same time. Many of us will have heard of radio waves or light waves for example, but sound and light can also be described as phonons (particles of sound) and photons (particles of light).
The uncertainty principle is applicable to many more particles, but we’ll use these as examples.
We can determine the momentum of a quantum object with a simple calculation. We measure the wave pattern between two peaks and get a number. This number is representative of momentum or how fast the object is travelling.
If we assume the above diagram is a light wave we could accurately determine momentum or speed simply by measuring the distance between two peaks in the wave pattern. We would need to have some unit of measurement represented on the horizontal scale of course, but we can assume there is one, whatever that might be.
However we would be absolutely at a loss to explain the position of a particle of light or photon as that is represented by each of the peaks on the wave pattern. It could be at any one of those peaks and we have no way of figuring out which one.
Lets look at another wave pattern:

Imagine the graph above (which is very much still a wave by the way) is describing the same thing. Now we can be certain where the photon particle is right? Absolutely, its at the position of the top of the peak, but how can we determine its momentum when don’t have any other wave peaks on the graph to measure between? The answer is we can’t.
By more accurately being able to determine position and having a single peak, we lose accuracy in being able to absolutely determine momentum.
By more accurately describing momentum and having many peaks to measure between, we lose accuracy in being able to absolutely determine position.
Whichever way we do it, there is always uncertainty over one or the other. In fact, increasing accuracy in one aspect decreases accuracy in the other.
This is Heisenberg’s uncertainty principle.
Practical implications
So, what does this mean for us in everyday life? In reality, very little as the uncertainty principle is only applicable to those very small quantum objects. It does however carry some wider philosophical meaning and underpins aspects of technology development.
Prohibiting knowledge of the exact position and momentum of a quantum object at the same time means nothing in our universe can ever be static. It must always be moving.
It does also appear to imply that its impossible to be completely sure of every aspect of that universe.
As emerging technologies utilise quantum mechanics more frequently the uncertainty principle is sure to turn up in your everyday life even if you don’t realise it.
For example ‘quantum communication’ is a method where un-hackable messages can be sent between computers. This is possible because the messages are actually carried by photons and as per the example above, any attempt to eavesdrop or decode them in transit would force a disturbance due to the measurement restrictions of the uncertainty principle.
Probably the biggest philosophical question would be to ask why this uncertainty is woven into the fabric of our universe in the first place?
Breaking Bad
In the TV show, Walt was a chemist and he knew his particle physics that’s for sure.
He opted to nickname himself ‘Heisenberg’ because he believed he was always one step ahead of the DEA, who would always have a degree of uncertainty over his whereabouts and consequently find it impossible to locate him precisely for an arrest.
Quite an attitude from a once meek mannered High School teacher!
I’m the founder of The Average Scientist and also an Astrophysicist, a passionate Science Communicator and elected Fellow of the Royal Astronomical Society.
I regularly speak at various events, including our TAS Talks and theatre shows on subjects such as Astrophysics, Planetary Science and the Evolution of the Universe.









1 Comment
[…] location in space. The wave-like and particle-like traits of a photon trade off according to the Heisenberg Uncertainty Principle. This means that the more you force a photon to act like a particle, for instance by confining it […]