Reading Time – 10 Minutes, Difficulty Level 1/5
The periodic table of elements, a masterpiece of scientific organisation, stands as a testament to human ingenuity and the unending quest to understand the fundamental building blocks of the universe. This iconic chart, with its 118 known chemical elements, offers more than just a visual representation; it unveils the profound patterns and relationships that govern the properties of these elements. While it may appear cryptic to the uninitiated, this article aims to delve deeply into the history, evolution, and underlying principles of the periodic table.
Defining the Elements
At the core of this grand structure are the elements themselves, the very essence of matter. An element is a substance composed of identical atoms, characterised by the number of protons within its nucleus, a value known as the atomic number. These atoms, with their positively charged protons and negatively charged electrons, form the fundamental units of all matter. For instance, an atom with 6 protons is unequivocally carbon, while an atom with 8 protons is unmistakably oxygen. Currently, our knowledge encompasses 118 elements, each represented by one or two-letter symbols, from hydrogen (H) to oganesson (Og).
Early Attempts at Organisation
The quest to organise these elements began in the early 1800s when scientists initially grouped elements based on their relative atomic weights. Yet, it wasn’t until the 1860s that Russian chemist Dmitri Mendeleev made a groundbreaking breakthrough. He arranged the 63 known elements in order of increasing atomic weight and observed a striking periodicity in their properties. Some elements shared similar chemical behaviours and reactivity, leading him to create vertical columns known as groups and horizontal rows known as periods. Mendeleev’s genius even allowed him to predict the properties of elements yet to be discovered, like gallium and germanium.
Around the same time, English chemist John Newlands proposed his “Law of Octaves,” grouping elements based on atomic weight, noticing that every eighth element exhibited similar properties. This early concept of periodicity set the stage for further exploration.

Image: Linda Hall Library, Kansas City, MIssouri. USA.
Transition to the Modern Periodic Table
In 1913, physicist Henry Moseley made a pivotal discovery that reshaped the periodic table’s foundation. He demonstrated that an element’s chemical properties and identity were determined by its atomic number, not atomic weight. This revelation allowed for the precise organisation of elements based on the number of protons within their nuclei.
Additional refinements followed, with Glenn Seaborg repositioning some elements and grouping transition metals in the middle section. The modern periodic table emerged, with metals on the left, nonmetals on the right, and metalloids bordering the line that separates them.
By the mid-1900s, the periodic table had taken the form we recognise today, with all 118 elements meticulously sequenced according to their atomic numbers. The table’s architecture now intertwined with the quantum physics principles underlying an element’s electron configuration.
Key Sections and Details of the Modern Periodic Table
The modern periodic table features 118 confirmed elements organised into 18 vertical columns known as groups and 7 horizontal rows known as periods. Reading from left to right and top to bottom, the elements are arranged in increasing atomic number order. Some essential features include:
- Groups: Elements within a group share common properties and have the same number of valence electrons.
- Periods: Each period corresponds to the principal energy level quantum number of an element’s electron shell configuration.
- Main Body: This area contains groups 1 (alkali metals) through 8 (noble gases).
- Transition Metals: These elements occupy the central portion of groups 3-12.
- f-Block: Lanthanides and actinides form the two rows at the bottom.
- Nonmetals: They are situated on the right side, marked by different coloured blocks.
- Reactive Metals: Found on the far left, they are among the most reactive elements.
- Metalloids: These semimetals border the zigzag line, separating metals from nonmetals.
The Staggering Brilliance of the Periodic Table
The periodic table’s elegant design reveals enlightening patterns related to electron configurations, providing crucial insights into atomic structure. Its periodicity stems from elements with similar properties recurring at regular intervals. While Dmitri Mendeleev pioneered the earliest version of the periodic table, it has seen numerous refinements over a century to reach its current form.
Continued discoveries, such as the recent addition of superheavy elements like nihonium, mark significant milestones. Ongoing research continues to push the boundaries of atomic knowledge, expanding our understanding beyond element 118.
Atomic Structure Insights Revealed
The periodic table serves as a powerful tool for studying atomic structure, offering insights into how electrons are arranged within an element’s atoms. The rows represent the filling of electron shells and subshells, while the columns reflect the successive filling of electron orbitals. This arrangement provides rich information about atomic configurations and behaviours.
Furthermore, the table sheds light on how atoms gain or lose electrons during chemical reactions, aiming to attain stable electron configurations. Understanding these fundamental concepts helps explain an element’s position and properties within the table.
Why the Periodic Table Matters
The periodic table stands as one of the most remarkable achievements in the history of science, providing a comprehensive framework for classifying elements while revealing their periodic behaviours. Although initially organised by atomic weight, subsequent breakthroughs shifted the focus to atomic number, setting the stage for the modern periodic table.
While Mendeleev’s contributions were groundbreaking, many brilliant minds over more than a century have refined this scientific masterpiece. Its organisation beautifully aligns with the quantum mechanical description of atomic structure and electron configurations.
As a scientific showpiece, the periodic table continues to guide research into the atomic and subatomic realms. Its patterns signify hidden periodicity resulting from electrons and atomic structure, offering insights into the nature of matter itself.
The Ever-Evolving Periodic Table
The periodic table’s evolution has been ongoing for over 150 years. A testament to the relentless pursuit of knowledge about the elements. What began as a basic ordering system based on atomic weight has transformed into a dynamic framework shaped by quantum mechanics, subatomic particles, and nuclear physics.
Dmitri Mendeleev’s original table left gaps to accommodate undiscovered elements, a practice that continues today. The periodic table continues to grow as humanity’s understanding of the elemental world expands.
In conclusion, the periodic table stands as an adaptable distillation of collective scientific discovery, capturing the quest to unravel the mysteries of the atomic and subatomic realm. Scientists expect it will continue to evolve as deeper insights emerge. Its enduring significance cannot be overstated, as it serves as a symbol of humanity’s pursuit of knowledge and understanding.
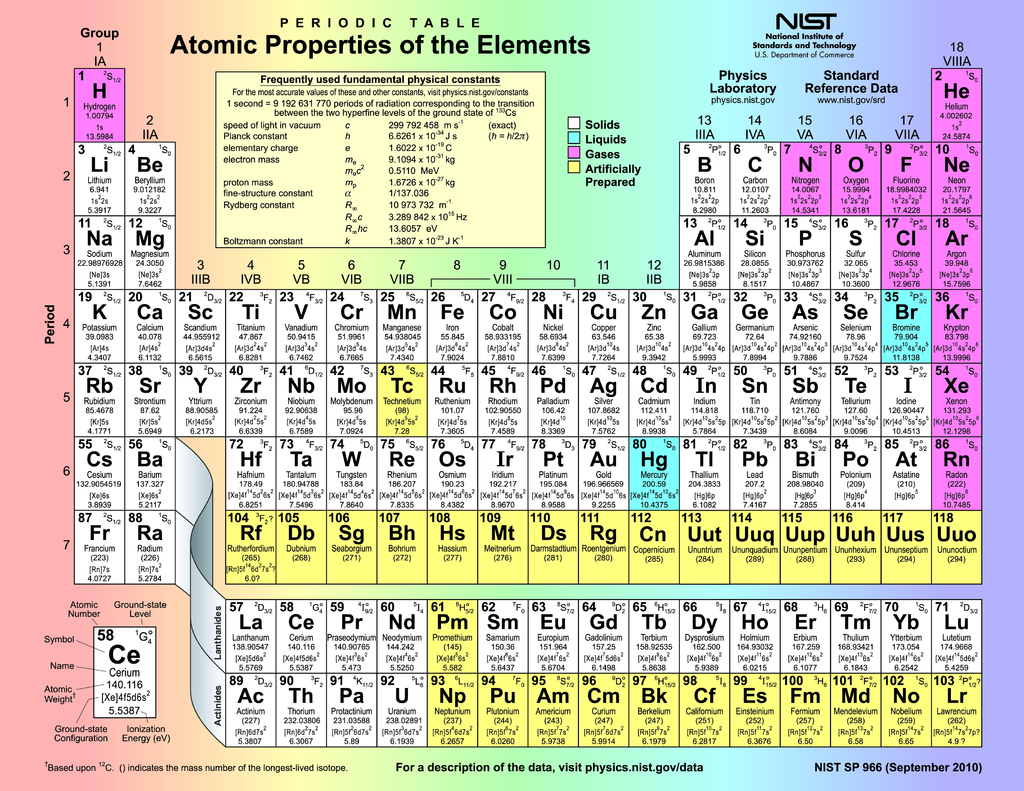
Beautiful, modern periodic table. R.A. Dragoset, A. Musgrove, C.W. Clark, and W.C. Martin — NIST, Physical Measurement Laboratory, Public domain, via Wikimedia Commons
The Role of the Periodic Table in Modern Science
The periodic table, a cornerstone of chemistry, has also found its place in various scientific disciplines. Its influence extends far beyond the realm of chemical reactions and properties; it serves as a fundamental tool in the study of diverse scientific fields.
Astrophysics and Cosmology
Even in the vast reaches of the cosmos, the periodic table plays a pivotal role. Spectroscopy, the study of light emitted or absorbed by matter, allows astronomers to determine the composition of celestial bodies. By analysing the spectral lines produced by elements, scientists can identify the elements present in stars, galaxies, and nebulae. This astronomical application of the periodic table has profound implications for our understanding of the universe’s composition and evolution.
Materials Science and Engineering
In the realm of materials science, the periodic table serves as a guide for discovering and designing new materials with specific properties. Engineers and scientists rely on this framework to select elements and create innovative materials for applications ranging from electronics to aerospace. The periodic table’s organisation facilitates the development of materials with tailored properties, revolutionising industries and technology.
Environmental Science and Sustainability
The periodic table also plays a crucial role in environmental science. Understanding the behaviour of elements and their compounds in natural ecosystems is essential for addressing environmental challenges. From studying the impact of heavy metals in soil and water to monitoring pollutants in the atmosphere, the periodic table provides a framework for comprehending the behaviour and interactions of elements in the environment.
Medicine and Pharmaceuticals
In the field of medicine and pharmaceuticals, the periodic table informs drug development and research. Medicinal chemists use their knowledge of elements and their properties to design molecules that target specific biological processes. Elements like carbon, hydrogen, nitrogen, and oxygen form the backbone of many pharmaceutical compounds, highlighting the intimate connection between chemistry and medicine.
Nuclear Physics and Energy
The periodic table’s role extends to the study of nuclear physics and energy production. Researchers in these fields explore the behaviour of elements at the atomic and subatomic levels. The understanding of isotopes, elements with the same number of protons but different numbers of neutrons, is essential for applications in nuclear reactors, radiological medicine, and nuclear weapons proliferation detection.
Education and Scientific Communication
Beyond its scientific applications, the periodic table serves as an educational tool that introduces students to the world of chemistry and the elements. Its visual representation simplifies complex concepts and fosters an appreciation for the natural world’s underlying order. Additionally, the periodic table’s structure aids in scientific communication, providing a common language for researchers and educators across the globe.
Recent Advances and Future Prospects
As scientific research advances, the periodic table continues to evolve. Recent developments include the synthesis of superheavy elements beyond oganesson (Og), with element 118 being the most recent addition. These discoveries push the boundaries of atomic knowledge and challenge our understanding of the stability and properties of such heavy nuclei.
Future prospects for the periodic table include the search for the “island of stability,” a theoretical region of superheavy elements that may exhibit significantly longer half-lives than currently known. Discovering and understanding elements within this region could have profound implications for nuclear physics and our understanding of the fundamental forces of the universe.
Additionally, advancements in technology, such as high-energy particle accelerators and improved spectroscopic techniques, will likely contribute to the discovery of new elements and the characterisation of their properties.
Final Thoughts
The periodic table, a symbol of scientific achievement and human curiosity, continues to shape our understanding of the natural world. From its humble beginnings as a simple arrangement of elements by atomic weight, it has transformed into a dynamic, adaptable framework that spans multiple scientific disciplines.
Its impact reaches far and wide, from the furthest reaches of the cosmos to the tiniest subatomic particles. Whether in astrophysics, materials science, environmental studies, medicine, or nuclear physics, the periodic table remains a steadfast companion in the pursuit of knowledge and innovation.
As we peer into the future of scientific discovery, the periodic table stands as a testament to the enduring human quest for understanding and serves as a reminder that the mysteries of the universe are waiting to be unveiled, one element at a time.
I’m the founder of The Average Scientist and also an Astrophysicist, a passionate Science Communicator and elected Fellow of the Royal Astronomical Society.
I regularly speak at various events, including our TAS Talks and theatre shows on subjects such as Astrophysics, Planetary Science and the Evolution of the Universe.








